Qualitative QC Micro Organism Formats
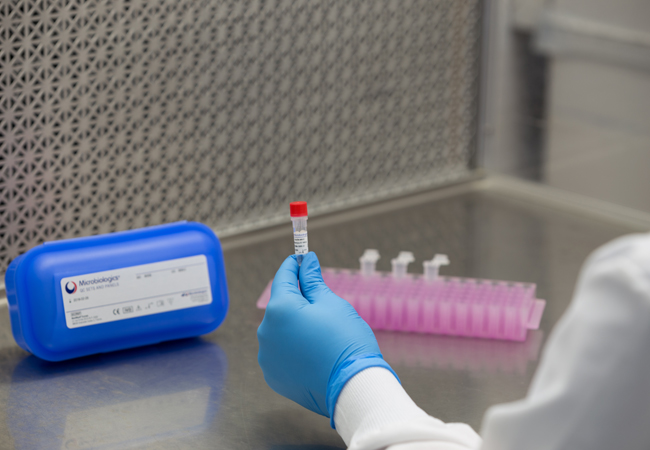
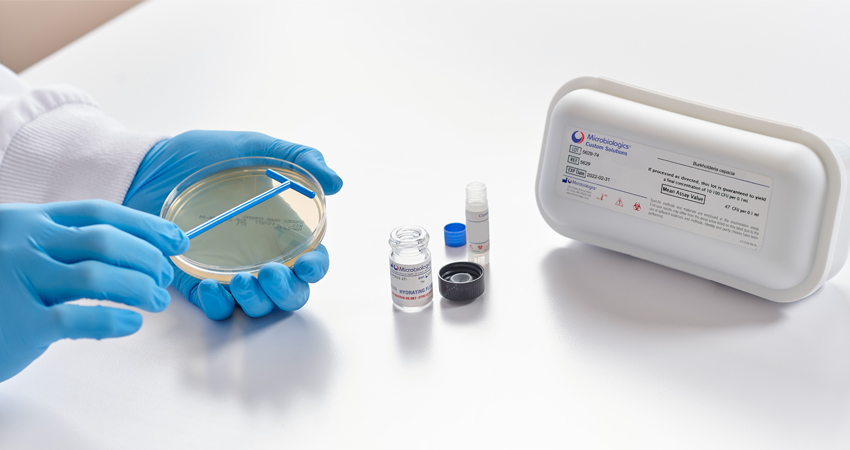
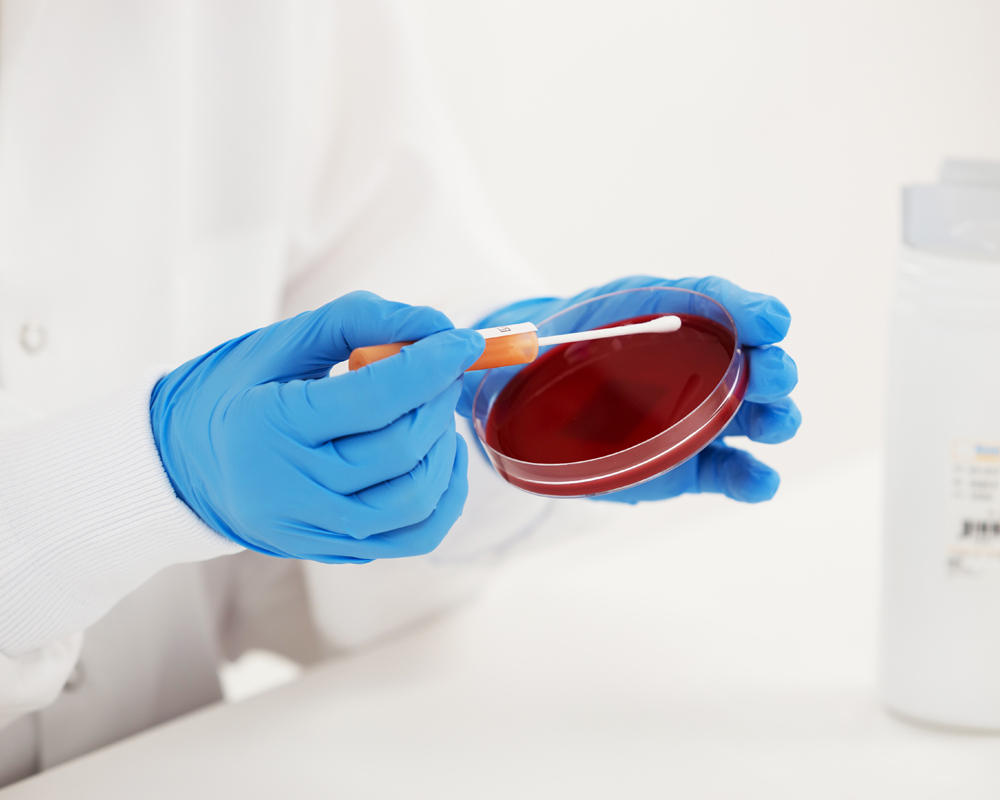
KWIK-STIK™, KWIK-STIK™ Plus and LYFO DISK™ microorganisms are intended to be used as controls to verify the performance of assays, reagents or media that are intended to be used in microbial testing for the detection and identification of a cultured microorganism isolate. All reference material is accredited under ISO 17034 standard, cultures are traceable to reference cultures to ensure authenticity and packages are refrigerated for easy and economical storage.
KWIK-STIK Product Sheet English LYFO DISK, KWIK-STIK, KWIK-STIK Plus Instructions For Use